Document distributed under permission of Rick Strassman MD
I. Neuroendocrine, Autonomic, and Cardiovascular Effects
Rick J. Strassman, MD, Clifford R. Qualls, PhD
BACKGROUND: To begin applying basic neuropharmacological hypotheses of hallucinogenic drug actions to humans, we generated dose-response data for intravenously administered dimethyltryptamine fumarate’s (DMT) neuroendocrine, cardiovascular, autonomic, and subjective effects in a group of experienced hallucinogen users.
METHODS: Dimethyltryptamine, an endogenous mammalian hallucinogen and drug of abuse, was administered intravenously at 0.05, 0.1, 0.2, and 0.4 mg/kg to 11 experienced hallucinogen users, in a double-blind, saline placebo-controlled, randomized design. Treatments were separated by at least 1 week.
RESULTS: Peak DMT blood levels and subjective effects were seen within 2 minutes after drug administration, and were negligible at 30 minutes. Dimethyltryptamine dose dependently elevated blood pressure, heart rate, pupil diameter, and rectal temperature, in addition to elevating blood concentrations of ß-endorphin, corticotropin, cortisol, and prolactin. Growth hormone blood levels rose equally in response to all doses of DMT, and melatonin levels were unaffected. Threshold doses for significant effects relative to placebo were also hallucinogenic (0.2 mg/kg and higher). Subjects with five or more exposures to 3, 4-methylenedioxymethamphetamine demonstrated less robust pupil diameter effects than those with two or fewer exposures.
CONCLUSIONS: Dimethyltryptamine can be administered safely to experienced hallucinogen users and dose-response data generated for several measures hypothesized under serotonergic modulatory control. Additional studies characterizing the specific mechanisms mediating DMT’s biological effects may prove useful in psychopharmacological investigations of drug-induced and endogenous alterations in brain function.
(Arch Gen Psychiatry. 1994; 51:85-97)
![]()
Hallucinogenic drugs reliably induce a unique constellation of subjective effects in humans. They elicit perceptual illusions and hallucinations, primarily visual but often auditory. Mood effects range from euphoria to panic to bland indifference. Somatic effects include dissociation, relaxation, or tension.1 “Classic” hallucinogens include phenethylamines (eg, mescaline), indolealkylamines (eg, psilocybin and dimethyltryptamine [DMT]), and the lysergamides (eg, lysergic acid diethylamide [LSD]).2
These unique compounds were the focus of intensive clinical research in the 1950s and 1960s. The discovery of LSD may arguably have been as important to the development of a “biological” psychiatry as was the contemporaneous discovery of chlorpromazine. Hallucinogens’ use in clinical research was associated with acceptable risks when subjects, both psychiatric patients and normal controls, were carefully screened, prepared, and followed-up.3 Clinical research with hallucinogens, however, became increasingly difficult with their widespread abuse by young adults. Highly publicized “adverse reactions” to hallucinogens, primarily LSD,4 resulted in their placement into the restrictive schedule I of the Controlled Substances Act. Human studies with these drugs effectively ended in the mid-1970s despite legal restrictions and severe penalties for manufacture, possession, and distribution of hallucinogens, their use among college students has held relatively steady over the past 20 years.5
Basic research into the mechanisms of action of hallucinogens continued, however, and contributed to recent advances in serotonin (5-HT) receptor pharmacology.6 Recent behavioral, in vivo, and ligand binding data suggest agonist or partial agonis effects at the 5-HT2, 5-HT1A, and 5-HT1C receptors.7-10 Dopaminergic11 and noradrenergic12 effects have been described but not studied recently.
Hallucinogenic drugs have diffuse and relatively well documented biological effects, presumably related to their serotoninergic properties. Growth hormone (GH), prolactin, ß-endorphin, corticotrophin, and cortisol levels are all affected by serotoneric stimuli.13 Consistent with the presumed serotonergic properties of the classic hallucinogens, levels of several of these hormones increase with their administration. For example, human prolactin blood levels rise in response to dimethyltryptamine14 and rat plasma corticoid levels are stimulated by LSD.15 Cardiovascular variables, core temperature, and papillary diameter also are affected by serotonergic hallucinogens. Examples of these effects include DMT’s hypertensive and mydriatic,16 LDS’s pyretogenic and mydriatic, 17, 18 and psilocybin’s hypertensive and tachycardic19 properties.
Dimenthyltryptamine is a short-acting hallucinogen, first discovered in hallucinogenic Amazonian snuffs.20 It was later synthesized and determined to be active by parenteral (intramuscular [IM] administration only.16 Its discovery in human body fluids prompted a flurry of investigations into its possible biosynthesis in man and its role as a putative endogenous psychotogen.21 difficulties distinguished peripheral levels of DMT between normal controls and patients with endogenous psychoses led to a loss of interest in the function of this hallucinogenic tryptamine.22
It has been impossible to test in humans hypotheses of hallucinogen mechanisms of action developed from basic research. Systematic psychopharmacological investigations are the necessary interface bridging recent neuropharmacological findings to human effects. Dose-response investigations applying current psychiatric research methods to assess hallucinogens’ effects can generate data providing the bases for more experimental studies, such as selective blockade with receptor sub-type antagonists.
Several properties recommend DMT as an appropriate drug with which to renew such investigations. Its short duration minimizes prolonged dysphoric reactions in the potentially stressful environment of a modern clinical research center. The lack of a widespread “folklore” concerning its effects provides less bias and expectations from subjects. Finally, DMT’s role in human brain function has yet to be explicated in normal and pathological states. That is, understanding effects and mechanisms of action of DMT may shed light on the etiology and treatment of endogenous hallucinatory conditions.
We have performed a dose-response study of the neuroendocrine, cardiovascular, autonomic, and subjective effects of dimethyltryptamine fumarate using a double-blind, placebo-controlled, randomized design. This article focuses on biological data generated by this study.
VIEW THE “DEMOGRAPHIC CHARACTERISTICS OF SUBJECTS” DIAGRAM.
BY CLICKING HERE.
RESULTS
The major findings of this study are the following: (1) Intravenous dimethyltryptamine fumarate was fully hallucinogenic in doses of 0.2 and 0.4 mg/kg. Effects were felt nearly instantaneously, peaked within 2 minutes after injection, and resolved within 20 to 30 minutes. The time course of DMT blood levels matched the march of subjective effects. (2) Multiple biological variables rose dose dependently in response to DMT. The time course of elevations in blood levels of pro-opiomelanocortin (POMC) peptides (corticotrophin and ß-endorphin), HR, blood pressure, and pupil diameter paralleled subjective effects and DMT blood concentrations. Elevations of prolactin and cortisol blood levels lagged 5 to 10 minutes behind these changes. Core temperature and GH effects did not begin until the first set of perturbations had almost resolved (15 to 30 minutes) and may not have peaked by 60 minutes after DMT administration.
Article continued on next page (links below).
DMT BLOOD LEVELS
There was no measurable DMT in blood samples before drug administration or after saline placebo administration for any subject except for subject 4. He had measurable DMT levels at one point before active drug administration and twice after placebo injection. His low levels in these circumstances are consistent with endogenous levels in a study of normal volunteers using a similar assay.30 However, he regularly took “Chinese herbs” and “homeopathic tinctures” as tonics, unlike any of our other subjects.
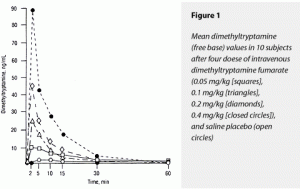
Plasma levels of DMT peaked at the first blood draw, 2 minutes after the injection was completed, closely corresponding to peak psychological effects. Generally, a doubling of dose resulted in a doubling of ∆Max values. These data are displayed in Figure 1. As previously described.31 DMT levels varied widely among subjects, peak values ranging from 32 to 204 ng/mL after the 0.4- mg/kg injection.
BASELINE EFFECTS
There were no differences among baseline values for any biological variable among the five treatment conditions.
NEUROENDOCRINE EFFECTS POMC PEPTIDES
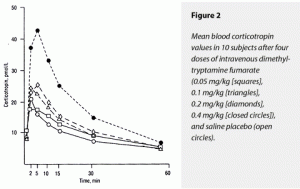
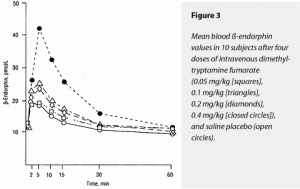
The ∆AUC and ∆Max for both corticoropin and ß-endorphin rose dose dependently in response to dimethyltryptamine fumarate, with the 0.4-mg/kg dose’s effects being statistically greater than all other doses for both of these variables. The effects of placebo and the 0.05- and 0.1-mg/kg doses could not be separated statistically. Of interest was the stimulation of corticotrophin and ß-indorphin levels by saline placebo. This has the appearance of a conditioned response to placebo injection in all these subjects who had previously received the somewhat anxiety-provoking 0.4-mg/kg dose nonblind. These data are displayed graphically in Figure 2 (corticotrophin) and Figure 3 (ß-endorphin).
Prolactin
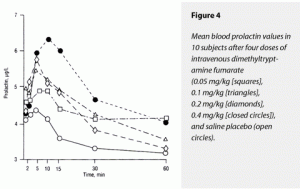
The ∆Max and ∆AUC for prolactin demonstrated a significant dose-dependent stimulatory effect of dimethyltryptamine fumarate. The 0.4-mg/kg dose raised both of these variables significantly more than placebo and the 0.05- and 0.1-mg/kg doses. The effects of 0.4 and 0.2 mg/kg on ∆Max prolactin could not be distinguished from each other, although the 0.4-mg/kg dose’s effects on ∆AUC were significantly greater than those of 0.2 mg/kg. The effects of 0.2, 0.1, and 0.05 mg/kg of dimethyltryptamine fumarate and of placebo on ∆AUC were not statistically separable. These data are graphically presented in Figure 4. Note that prolactin effects were slightly delayed relative to the POMC peptides.
Cortisol
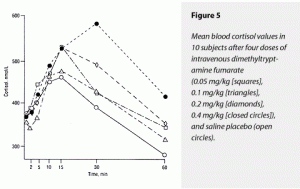
The ∆AUC and ∆MAX for cortisol both demonstrated dose-dependent stimulatory effects of dimethyltryptamine fumarate. The 0.4-mg/kg dose raised these two variables more than all other doses. The effects of 0.4 mg/kg on ∆AUC for cortisol could not be statistically distinguished from the effects of 0.2 mg/kg, however. Neither could the 0.4-mg/kg dose’s effects on ∆MAX for cortisol be separated statistically from 0.2 and 0.05 mg/kg. Effects of the 0.05, 0.1-, and 0.2-mg/kg doses on ∆AUC and ∆MAX were not statistically different, although their effects were numerically greater than those of saline. These data are graphically presented in Figure 5.
Growth Hormone
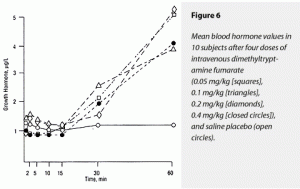
There was a trend for DMT to produce a rise in ∆AUC for GH. The ∆MAX for GH rose in response to DMT administration. Although all doses of DMT raised GH levels relative to saline placebo; effects of different doses could not be distinguished from each other. These data are graphically displayed in Figure 6. Note the delay in GH effects relative to the other neuroendocrine results.
Melatonin
We only assayed six subjects’ blood samples for melatonin from their 0.4-mg/kg sessions. Utilizing one-sample t tests, there was no effect of 0.4 mg/kg of dimethyltryptamine fumarate on either ∆MAX or ∆AUC for melatonin (data not given), so the remaining samples were not assayed.
AUTONOMIC EFFECTS
Rectal Temperature
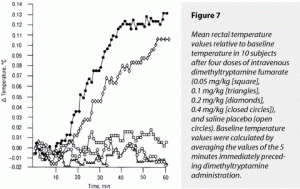
The ∆MAX and ∆AUC for temperature demonstrated a significant effect of dimethyltryptamine fumarate. For ∆AUC, the 0.4-mg/kg dose’s effects were greater than those of 0.1 and 0.05 mg/kg and saline, but could not be distinguished from those of 0.2 mg/kg. The effects of 0.2, 0.1, and 0.05 mg/kg of drug and of saline could not be statistically separated. For ∆MAX in temperature, the 0.4- and 0.2-mg/kg doses’ effects were greater than those produced by 0.1 and 0.05 mg/kg or placebo. The ∆MAX effects of 0.4 and 0.2 mg/kg could not be statistically separated from each other; neither could the effects of the three latter treatments be separated. These data are graphically displayed in Figure 7, where they delayed effect relative to other variables can be seen.
Pupil Diameter
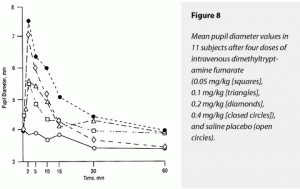
As discussed in the “Procedures” section, our data for pupil diameter contained the most missing points and should be interpreted cautiously. Missing values made a calculation of ∆AUC pupil diameter impossible for the more disruptive 0.2- and 0.4-mg/kg doses. Furthermore, these data were analyzed using ANOVA without repeated measures, a less robust analytic tool.
The ∆MAX pupil diameter demonstrated significant effects of DMT. The 0.4-mg/kg dose increased pupil diameter more than all other treatments. Effects of placebo and 0.05, 0.1, and 0.2 mg/kg could not be distinguished from each other. These data are displayed graphically in Figure 8.
Article continued on next page (links below).
CARDIOVASCULAR EFFECTS
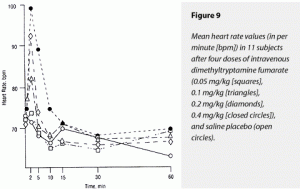
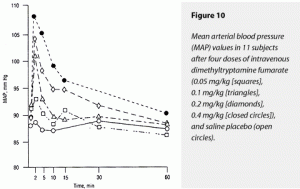
Dimethyltryptamine dose dependently elevated ∆MAX and ∆AUC values for both HR and mean arterial blood pressure (MAP). For ∆MAX HR, effects of 0.4 and 0.2 mg/kg were significantly greater than those of 0.1 and 0.05 mg/kg and saline; these latter three treatments’ effects were not distinguishable from each other. Neither were the effects of 0.4 and 0.2 mg/kg separable from each other. Effects on ∆AUC HR were less clearly separable by dose but followed the same pattern
The 0.4-mg/kg dimethyltryptamine fumarate dose raised ∆AUC MAP significantly more than 0.1 and 0.05 mg/kg and saline. These latter three treatments’ effects could not be distinguished from each other. Neither were the effects of 0.2 and 0.4 mg/kg statistically separable. The 0.4- and 0.2-mg/kg doses caused significantly greater elevations of ∆MAX MAP than 0.1 and 0.05 mg/kg and saline; the two former doses’ effects could not be distinguished from each other. These data are graphically displayed in Figure 9 (HR) and Figure 10 (MAP).
MDMA EFFECTS
For all of the biological variables under investigation, only ∆MAX pupil diameter values were smaller across all doses of DMT in the high-exposure compared wit the low- or no- exposure MDMA group (high exposure, 0.6±0.2 mm; low or not exposure, 1.5±0.2 mm; F=11.3, P=.002).
SUBJECTIVE EFFECTS
A detailed description of subjective effects, and the development of and preliminary data generated by our new rating scale, the Hallucinogen Rating Scale (HRS), are presented in a companion article.32 Briefly, subjects felt the onset of effects before the 45-second infusion was completed. Peak hallucinogenic effects (with 0.2 and 0.4 mg/kg) occurred usually by the first blood draw and vital signs check, at 2 minutes after injection, a useful temporal reference point for subjects. Subjects remained moderately intoxicated for another 10 to 20 minutes. They felt relatively normal at 20 to 30 minutes after injection. Several described a relaxed, “at ease” feeling for an additional 30 minutes.
All subjects but one experienced visual hallucinatory phenomena at 0.2 and 0.4 mg/kg of dimethyltryptamine fumarate. Brief, poorly formed auditory hallucinations ere experienced by most subjects. Somatic effects consisted of an intense rush, felt throughout the body or specifically in the head, and bodily dissociation, usually associated with a transient anxious or fearful emotional reaction. Cognitive effects were not particularly profound; rather, an “observing ego” was maintained, alert and attentive to the perceptual and somatic effects of the drug. The 0.2-mg/kg dose’s perceptual perturbations, rush, and disorganizing in effects were less intense than for 0.4 mg/kg, and 0.2 mg/kg was the dose preferred by many subjects. The 0.1-mg/kg dose of dimethyltryptamine fumarate produced hallucinatory phenomena in only one subject (the woman in the study). Somatic effects predominated, and many subjects disliked this dose the most. The 0.05-mg/kg dose was mistaken for placebo by several subjects. If effects were noted, they were usually somatic, with a warm, relaxed, and “floating” bodily sensation.
—-COMMENT—-
We safely administered graded doses of IV DMT, and endogenous hallucinogen and schedule I drug of abuse, to a group of experienced hallucinogen users. Dose-dependent responses were observed for several neuro-endocrine, cardiovascular, and autonomic variables in a randomized, double-blind, saline placebo-controlled design. Peak psychological effects paralleled the time course of DMT blood levels, which appeared, peaked, and fell rapidly. Blood levels of the POMC peptides, corticotrophin and ß-endorphin, closely followed the course of DMT levels and hallucinogenic effects, as did pupil diameter and cardiovascular responses. Cortisol and prolactin levels rose in a dose-dependent fashion and followed the first group of variables’ course by 5 to 10 minutes. Core temperature and GH stimulation were most delayed, although GH effects were not separable by dose. Stimulation of daytime melatonin levels was not seen.
The threshold for significant biological effects of dimethyltryptamine relative to placebo was also that for its hallucinogenic properties: 0.2 mg/kg and higher. The 0.4-mg/kg dose of IV dimethyltryptamine fumarate produced blood levels and psychological effects comparable with those seen with administration of more than twice higher doses of IM dimethyltryptamine hydrochloride or creatine sulfate.31 However, the IV route generated a more rapid onset (nearly instantaneous) and offset for these effects than the IM route.
Dimethyltryptamine, found in Amazonian hallucinogenic plant mixtures and human body fluids, meets electrophysiological,33 pharmacological,34 and behavioral35 criteria for hallucinogenicity in lower animals. It had been used safely in humans in this country and Europe.14, 16, 31 Its short duration of action was believed capable of providing a discrete, more manageable hallucinogenic state in a clinical research setting than might the 8- to 12-hour effects of lysergic acid diethylamide.
Hallucinogenic drugs are serotonergic agonists or partial agonists, in addition to having adrenergic and dopamineric properties. The 5-HT receptor subtypes of interest include the 5-HT2/1C and 5-HT1A. 36, 37 We therefore chose our measured variables because they are believed regulated or modulated to a significant degree by serotonergic neurotransmission. These variables included multiple neuro-endocrine markers, blood pressure, HR, rectal temperature, and pupil diameter. As DMT has nearly equal affinities for both the 5-HT2 and 5-HT1A subtypes,34 many of the effects to be discussed may devolve from activation of either; however, functional interactions in humans also have been described 38 and may be important.
Levels of the POMC peptide hormones, corticotrophin and ß-endorphin, both rose dose dependently in our subjects. They rose quite quickly, peak values being seen at 5 minutes after injection. With doses of 0.1 mg/kg and higher, ∆AUC and ∆MAX values for these two hormones were nearly identical, confirming their corelease.39 These rises in ß-endorphin and corticotrophin extend human40, 41 data demonstrating serotonergic stimulatory effects. Either 5-HT1A 42 or 5-HT2 43 subtypes may be involved.
Prolactin levels rose in our subjects in a dose-dependent manner, confirming and extending the data of Meltzer et al,14 wherein cyproheptadine inconsistently blocked DMT’s stimulatory effect. Multiple experimental procedures44-47 have suggested a stimulatory effect of serotonergic neurotransmission on prolactin levels. Subtype delineation is not clearly understood, but both 5-HT1A 48 and 5-HT2 49 receptors appear involved.
Cortisol levels rose dose dependently in our subjects, consistent with the robust elevation of corticotrophin described above, and with earlier human data. 14 Serotonin receptor activation stimulates peripheral cortisol levels, most likely via corticotrophin release,50 with relevant subtypes having been discussed previously. The cortisol effects in our subjects did not provide as useful a differentiation among doses as the POMC hormones.
Hallucinogen-induced hyperthermia in humans51 may occur by means of central52 and/or peripheral55 5-HT2 activation. Dimethyltryptamine’s hyperthermic effects are probably mediated by the 5-HT2 subtype, as 5-HT1A agonists are hypothermic in man.42 The delayed time course of the temperature rise seen with DMT is of interest and may reflect more complex homeostatic mechanisms controlling this variable than those with a more rapid time course.
Previous human studies have described variable effects of longer-acting hallucinogenic drugs on cardio-vascular variables, including hypotension and hypertension, and tachycardia and bradycardia.54 Dimethyltryptamine’s excitatory cardiovascular properties confirm and extend previous human data55 and suggest 5-HT2 agonism. The 5-HT2 agonists usually elicit a rise in blood pressure, without reliable increases in HR.56, 57 In contrast, 5-HT1A agonists generally produce a drop in blood pressure, again without consistent HR effects.58
Dimethyltryptamine resembles other classic hallucinogens in its mydriatic properties59, this papillary dilatation may be 5-HT2 mediated.60 As the papillary diameter data were both the least reliably measured (ie, we did not use automated equipment, which also could have measured more dynamic features of pupillary function) and contained the most missing values, they must be considered preliminary.
We found no dose dependency of DMT’s stimulation of blood GH levels. Meltzer et al14 demonstrated a cyproheptadine-sensitive stimulatory effect of one DMT dose on peripheral GH levels in humans. Perhaps if we had continued drawing samples for 2 hours, as did Meltzer et al, we might have found better separation of effects by dose.
Subjective effects of IV dimethyltryptamine were consistent with previous reports of parenterally administrated DMT16, 31, 61 and were marked by perceptual, dissociative, affective, and cognitive alterations typical of the classic hallucinogens. Onset from the IV route was nearly instantaneous, rather than taking several minutes to develop, and effects were shorter than previously published IM data. However, this time course is quite similar to reports of DMT smoked free base.27 The three subjects in the group with experience smoking DMT free base all volunteered that the IV route was somewhat more rapid and intense on onset and effects than smoking the compound. In this regard, DMT is actively transported across the blood-brain barrier in the rat.62 A more detailed account of these effects and their quantification by a new rating scale are described in a companion article.32
Dimethyltryptamine, as a putative directly acting 5-HT agonist in humans, can be most appropriately compared with 6-chloro-2 (l-piperazinyl) pyrazine (MK-212) and m-chlorophenylpiperazine (m-CPP), although only m-CPP has been given intravenously to humans. Neither drug produces the typical hallucinogenic syndrome in normal subjects; however, behavioral effects may be more disruptive in psychiatric samples.61-64 Both m-CPP and MK-212 have complex effects at multiple 5-HT receptor subtypes, in addition to effects on other neurotransmitters.65
Intravenous m-CPP’s biological effects are similar to those of DMT. Cortisol and GH stimulation is comparable in magnitude to DMT’s but the prolactin response to DMT is smaller.66 Similarly, GH responses are delayed relative to those of prolactin and cortisol. Hyperthermic effects of IV m-CPP also are relatively delayed and are greater than those seen with DMT.67 However, temperature data were collected longer for IV m-CPP than for DMT, and we may have missed a rise of similar magnitude. Behaviorally, IV m-CPP’s peak effects occur somewhat later than those of DMT.67 perhaps related DMT’s previously mentioned active transport into the brain.
Orally administered MK-212 elevates prolactin and cortisol (but not GH) blood levels,64 blood pressure, and body temperature.68 As MK-212 has never been given intravenously to humans, a comparison of the temporal sequence of its effects with IV DMT or m-CPP is not possible.
The only biological effect demonstrated in subjects with a greater cumulative exposure to the serotonergic neurotoxin MDMA relative t the group with less exposure was a decreased papillary response to all doses of DMT. This is opposite to what might be expected based on augmented behavioral effects of hallucinogens in amine-depleted69 or denervated70 animals. We are unable to explain this finding, except to note that our pupil diameter data were the least complete and objectively measured, and were analyzed by ANOVA without repeated measures.
We erroneously hypothesized that daytime melatonin levels might be elevated secondary to DMT administration, based on the presence of 5-HT2 receptors on pineal tissue71 and stimulation by DMT of a requisite enzyme for melatonin synthesis.72 However, that lack of a stimulatory effect of DMT on melatonin is relevant regarding the specificity of our neuroendocrine results. Previous work73 showed that melatonin levels doubled after a “stressful” long-distance race, although ß-endorphin levels rose to only half those noted in the present DMT study. These two sets of data make it unlikely that a “stress” effect (ie, purely sympathetic activation) drove our neuroendocrine variables; if so, either melatonin levels would have risen or ß-endorphin effects would have been much greater.
Article continued on next page (links below).
CAVEATS
Our sample of subjects had a heavy loading of personal (50%) and family (67%) histories of affective disorder and/or alcohol abuse, both of which may be associated with abnormalities in serotonergic responsivity.74, 75 Thus, the data generated from this study may not be generalizable to strictly “normal” subjects. In addition, nine of 11 subjects had been divorced, there being a total of 17 divorces in this group. Although not specifically assessed, this high rate of divorce may imply a certain degree of impulsivity within our subjects, another condition in which serotonergic abnormalities have been described.76
One subject’s blood pressure response to a screening, nonblind dose of 0.04 mg/kg caused him to be withdrawn from further participation. After completing this study, while screening additional subjects for subsequent protocols, another subject with a history of borderline essential hypertension (currently asymptomatic) also had a robust diastolic response to 0.05 mg/kg of dimethyltryptamine fumarate as was withdrawn from further sessions. Thus, we recommend that subjects with a history of hypertension, even though currently asymptomatic, not be considered for IV DMT studies. In addition, all prospective subjects must first be screened with the low, minimally psychoactive 0.05-mg/kg dose; diastolic blood pressure responses to more than 100 mm Hg contraindicate exposure to higher doses of DMT.
—-CONCLUSION—-
Human data are necessary to begin bridging basic neuropharmacological and clinical psychopharmacological concepts of hallucinogenic drug’s effects and mechanisms of action.77 Using careful screening and preparation of subjects, attentive yet unobtrusive supervision of the acute drug intoxication, and follow-up, DMT was safely administered to a group of experienced hallucinogen users and normative dose-response data generated. The most salient biological variables, based on our data, seem to be corticotrophin, prolactin, core temperature, blood pressure, HR, and pupil diameter. Pupil diameter studies, if performed, would benefit from the use of automated equipment.
We believe the importance of these data derive from at least two perspectives. The first is that of understanding effects and mechanisms of action of these intriguing drugs. Many of DMT’s biological effects in psychiatrically healthy hallucinogen users are now much better characterized. The properties of other classic and nonclassic hallucinogens (such as 1-(2, 5-dimethoxy-4 methylphenyl)-2-aminopropane (DOM) now can be assessed for their degree(s) of similarity. In addition, although serotonergic mechanisms are believed primary in mediating hallucinogens’ effects, these data can help validate this hypothesis by performing human studies using selective 5-HT receptor subtype antagonists. Experiments also can be designed to assess the relative contributions of noradrenergic and dopaminergic neurotransmission.
Second, DMT might be considered an additional serotonergic probe of neuroendocrine and behavioral variables in research attempting to characterize neurotransmission in normal volunteers and psychiatrically ill subjects with presumed serotonergic abnormalities. Although the hallucinogenic effects of DMT are not separable from its threshold effects (relative to placebo) on the multiple biological factors presented herein, these effects, if judiciously managed, should not present insurmountable difficulties. M-CPP, for example, increases several patient populations’ symptoms but remains a valuable tool for human serotonergic studies. However, it must be emphasized that until DMT’s effects are mechanistically characterized in normal subjects, there would not be sufficient data to conclude that altered responses to DMT necessarily implicated altered 5-HT function.
![]()
SUBJECTS AND METHODS
The protocol was approved by the Scientific Advisory Committee of the General Clinical Research Center (GCRC) and the Human research review Committee of the University New Mexico School of Medicine, Albuquerque, the New Mexico State Pharmacy Board, the US Drug Enforcement Administration, and the US Food and Drug Administration.23 Witnessed written informed consent was obtained from all subjects. Confidentiality and anonymity were maintained throughout the study.
SUBJECTS
We recruited only experienced hallucinogen users. All subjects but one were recruited by word of mouth; this last subject responded to an announcement sent to a graduate student training office at a local university.
Subjects were initially interviewed by one of us (R.J.S.) to screen for medical and/or psychiatric conditions and assess level of experience with, and ability to manage dysphoric reactions to, hallucinogenic drugs. Subjects were dripped from further consideration if they were taking any medications long-term, had a history of psychosis not secondary to drugs or fever, or had a chronic medical disorder.
Prospective subjects then received a Structured Clinical Interview for DSM-III-R (SCID-R), Outpatient Version,24 administered by a trained psychiatric research nurse. First degree relatives’ psychiatric histories were obtained during the SCID-R. Diagnoses were confirmed by discussion with two research psychiatrists. We then performed a medical history, physical examination, and laboratory screening tests (including a complete blood count with differential cell count, 24-item chemistry panel, thyroid functions [including thyrotropin], urinalysis, and electrocardiogram).
The demographic characteristics of our subjects are given in the Table. Subject 11 was dropped after receiving the non-blind (0.04-mg/kg) doses and the 0.05-, 0.1-, and 0.2-mg/kg doses because he had a major depressive episode precipitated by multiple personal stressors that appeared unrelated to his participation in the study. He responded to desipramine hydrochloride. The average (±SEM) age (excluding subject 11) was 41.5±1.5 years. The group was high functioning, with only one subject not being a professional or student in a professional training program. There also was a high incidence of prior major depression in the group. Only one subject was suffering any current Axis I disorder; this was an adjustment disorder resulting from an impending divorce. There also was a high incidence of first-degree relatives suffering from depression and/or alcohol abuse. The number of exposures to hallucinogens ranged from six to “hundreds.” Two subjects described a history of cocaine dependence/abuse, currently in remission; the remainder had little or no exposure to cocaine or amphetamines. Not all subjects had used marijuana equally, although all had some experience with it.
METHYLENEDIOXYMETHAMPHETAMINE USE
Of interest was the breakdown of subjects by amount of methylenedioxymethamphetamine (MDMA, Exstasy”) use. All but two had used MDMA at least once. Six of the subjects had used MDMA five or more times; five had used it twice or less. Methylenedioxymethamphetamine is a mammalian serotonergic neurotoxin25 with equivocal long-term effects on human neuroendocrine and/or behavioral functions.26 Thus, we were interested in assessing differential effects of the serotonergic agonist, DMT, in these two groups of users. For the sake of approximating equal cell sized, we described the six former subjects as having had high exposure and the latter five as having had low or no exposure.
DRUG
Dimehtyltryptamine fumatate was prepared by David E. Nichols, PhD, Purdue University, West Lafayette, Ind. Purity, determined by gas chromatography-mass spectrometry and high-performance liquid chromatography, was greater than 99.9%. It was prepared for clinical administration by the Inpatient Pharmacy of the University of New Mexico Hospital in vials containing 40 mg/mL. sterility and pyrogenicity testing revealed no abnormalities in a representative sample of vials. The dimethyltryptamine fumarate solution was drawn into a sterile tuberculin syringe at the appropriate dose and diluted with sterile saline to 1.0 mL before administration.
PROCEDURES
Non-blind Drug administration
First, all subjects received non-blind administrations of 0.04 and 0.4 mg/kg of intravenous (IV) dimethyltryptamine fumarate I the GCRC, usually on consecutive days. Previous work22 had demonstrated no tolerance to the cardiovascular and subjective effects of a full IM dose of DT given twice daily for 5 consecutive days. Thus, we did not believe tolerance to MDTs effects would occur at this interval. We decided to administer the drug IV rather than IM as had been reported previously.16 The onset and intensity of effect of 1.0 mg/kg of IM dimethyltryptamine fumarate was described by this subject as significantly less rapidly developing and hallucinogenic than his previous experience with the smoked drug. Further dose-finding studies with him and another experienced DMT smoker established that 0. mg/kg of IV dimethyltryptamine fumarate produced a “rush” and hallucinogenic effect comparable with those seen with a “full dose” of smoked free base.
The non-blind days served several purposes. They allowed us to assess the cardiovascular and subjective effects of a subclinical and fully hallucinogenic dose of dimethyltryptamine in a novel and potentially anxiety-provoking setting. These days also provided the research team and subjects an opportunity to become acquainted and familiar with each other in the research center setting without the extensive blood drawing and use of the rectal thermostat that could exaggerate any potentially paranoid reactions. Subjects also were able to “calibrate” themselves to what to expect regarding minimal and maximal effects of the drug, one of the purposes of the double-blind study being whether they could distinguish among incremental doses of DMT. Finally, these non-blind days provided subjects with the opportunity to drop out o the study before we had collected extensive data on them.
Subjects were admitted to the GCRC by 9 am after having fasted since midnight. An IV line was inserted into a forearm vein and kept patent with heparinized saline. Subjects were allowed to relax for 30 minutes before he drug was given. Dimethyltryptamine was infused over 30 seconds and flushed with 5 mL of sterile saline over the next 15 seconds. Blood pressure and heart rate (HR) were taken with an automatic cuff several times before and frequently during the 30 minutes following drug administration. The research team (a registered nurse and psychiatrist) sat quietly on either side of the subject, attentive to verbal and nonverbal cures. But did not offer any direction or advice unless absolutely necessary during the first several minutes, the time of the peak effect of the drug. Sublingual nitroglycerin, and IV diphenhydramine and diazepam, were available at the bedside for sustained hypertension, allergic reactions, or excessive anxiety, respectively. As drug effects resolved, discussion focused on the nature of the subject’s experiences. After subjects ate a snack or meal, they were discharged home.
No subject withdrew voluntarily after the non-blind days. One subject was withdrawn because of a marked diastolic blood pressure response (to >105 mm Hg) to the 0.04-mg/kg dose.
Double-blind, Placebo-Controlled, Randomized Drug Administration
A computer-generated randomization sequence was coded and kept by the Inpatient Pharmacy of the University of New Mexico Hospital. Subjects were admitted by 9 am to a dimly lit (<100 lux) room on the GCRC. Two IV Lines were started: a small-gauge, three holed plastic angiocatheter for blood withdrawal. A reusable rectal thermistor probe (YS-400, Yellow springs Instruments, Yellow Springs, Ohio), configured to a physiological monitor (Vita-Log PMS-8, CPS Inc, Palo Alto, Calif), was inserted. The monitor sampled rectal temperature every minute, and the thermistor probe has an accuracy of ±0,03°C. Blood pressure and HR (vital signs), and pupil diameter (measured by placing a card with black circles differing by 1-mm increments juxtaposed 10 to 15 cm from the subject’s eyes), were taken at 30, 15, and 2 minutes before drug administration. Blood samples for corticotrophin, ß-endorphin, prolactin, GH, cortisol, and melatonin levels were drawn 30 and 2 minutes before drug administration. Drug and saline flush were administered over 45 seconds, and time 0 was when the saline flush was completed. Doses were 0.05, 0.1, 0.2, and 0.4 mg/kg of dimethyltryptamine fumarate and sterile saline placebo. Blood samples were drawn, and pupil diameter and vital signs measured 2, 5, 10, 15, 30, and 60 minutes after drug administration. Study sessions occurred at an interval of at least 1 week and took place from January to September 1991. The one woman subject was studied during the early follicular phase of her cycle. A negative serum pregnancy test drawn the night before her study days was always available before drug administration.
Article continued on next page (links below).
ASSAYS
Dimethyltryptamine (free base) blood levels were assayed using the gas chromatography-mass spectrometry technique of Walker et al.28 Limit of detectability was 1 ng/mL. Corticotropin and ß-endorphin levels were assayed by immunoradiometric assays (Nichols Diagnostic Laboratories, San Juan Capistrano, Calif), and lower limits of detectability were 0.2 and 2.2 pmol/L, respectively. Limits of detectability for cortisol, GH, and prolactin radioimmunoassay (all from diagnostic Products, Los Angeles, Calif) were 6 nmol/L, 1 µg/L, and 1 µg/L, respectively. Growth hormones and prolactin values below 1 µg/L were entered as 0.9 µg/L. The melatonin radioimmunoassay29 limit of detectability was 5 pmol/L. All assays were performed in duplicate.
STATISTICS
Variable values are given as mean ±SEM. All analyses were performed using PCSAS Version 6.03 (Cary, NC). Several derived variables were computed for analyses. Baseline values for blood pressure, Hr, and pupil diameter were derived by averaging the three values obtained before drug administration (ie, -30, -15, and -2 minutes). Baseline temperature was computed by averaging values for 5 minutes immediately preceding drug administration. Hormone baseline values were computed by averaging results from the -30- and -2 minute samples. Maximum rise above baseline is indicated ∆Max; ∆AUC represents the area under the curve above baseline, calculated by the trapezoidal rule.
Our primary statistical tool was the univariate analysis of variance (ANOVA) with repeated measures, with dose as the repeated factor. Pairwise comparisons, when the overall F value generated a P<.05, were performed using the ‘contrast” statement in the general linear models procedure in SAS. To assess a possible modulatory role of methylenedioxymethamphetamine exposure on dimethyltryptamine’s effects on a particular variable, we performed a two-way univariate repeated-measures ANOVA using MDMA history as the independent variable.
MISSING DATA
We did not analyze blood hormone level results for the one female subject in the group because her veins collapsed at some point for almost every session, and he blood data were incomplete. However, we did analyze her autonomic ad cardiovascular responses.
Pupil diameter was difficult to measure consistently, as subjects varied widely in their ability and/or desire to open their eyes during acute drug intoxication. Thus, these measurements were obtained at the subjects’ discretion. Additionally, several subjects had technically poor rectal temperature tracings because of difficulties with probe placement, and these data could not be used. Two subjects had unsatisfactory temperature data for the 0.05- and 0.2- mg/kg sessions; one had a no 0.1 mg/kg session data; and one had not temperature data from his placebo session. Finally, one subject refused the rectal thermistor probe, and no temperature data were collected from him. As SAS cannot perform repeated measures ANOVA on subjects with missing data, we used a less robust one-way ANOVA for the temperature and pupil diameter data, with dose as the independent variable. Fisher’s Least Significant Difference Test for pairwise comparisons was performed if the overall F value generated a significance of P<.05. To assess the effect of DMT exposure on MDMA’s effects on pupil diameter and rectal temperature, we performed a two-way ANOVA with dose and MDMA history as independent factors.
![]()
Accepted for publication November 4, 1992
This investigation was supported by grant R03-DA06524 from the National Institute on Drug Abuse, Rockville, Md; the Scottish Rite Foundation for Schizophrenia research, NMJ, Lexington, Mass; grant RR0997-13 from the National Institutes of Health General Clinical Research Center, Rockville; the Scott Rogers fund of the University of New Mexico, Albuquerque; and University of New Mexico Department of Psychiatry research funds.
We thank Avid E. Nichols, PhD, for synthesis of the dimethyltryptamine fumarate used in this study; Cynthia Geist, RN, and the nursing staff of the GCRC, 5E, University of New Mexico Hospital, for nursing support; Katy Zownir-Brazis, RN, for performing the SCID-R interview; E. Jonathan Lisansky, MD, for assistance in diagnosis; Joy McLeod, Alberta Bland, and Susan Lee for laboratory assistance; David S. Schade, MD, for development of the dimethyltryptamine assay; and Richard Dorin, MD, and Russel Reiter, PhD, for assistance in performing assays.
Reprint requests to the Department of Psychiatry, University of New Mexico, 2400 Tucker Ave NE, Albuquerque, NM 87131-5326 (Dr. Strassman).
—-REFERENCES—-
1. Freedman DX. On the use and abuse of LSD. Arch Gen Psychiatry. 1968; 18: 330-347.
2. Nichols D. Oberlender R, McKenna D. Stereochemical aspects of hallucinogenesis. In: Watson R, ed. Biochemistry and Physiology of Substance Abuse, IIII. Boca Raton, Fla: CRC Press Inc; 1991; 1-39.
3. Cohen S. Lysergic acid diethylamide: side effects and complications. J Nerv ment Dis 1960; 130:30-40.
4. Strassman R. Adverse reactions to psychedelic drugs: a review of the literature. J Nerv Ment Dis. 1984; 172:577-595.
5. Pope H Jr. lonescu-Pioggia M, Aizley H, Varma D. Drug use and life style among college undergraduates in 1989: a comparison with 1969 and 1978. Am J Psychiatry. 1990; 147::998-1001.
6. Peroutka S, Snyder S. Mulitple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol, 1979; 16:687-690.
7. Glennon R, Titeler M, McKenney J. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1985; 35:2505-2511.
8. Spencer D Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N, N-dimethyltryptamine. Psychopharmacology. 1987; 93:158-166.
9. Yagaloff K, Hartig P. 125l-lysergic acid diethylamide binds to t novel serotonergic site on rat choroid plexus epithelial cells. J Neurosci. 1985; 5:3178-3183.
10. Buckholtz N, Zhou D, Freedman DX, Potter W. Lysergic acid diethylamide (LSD) administration selectively down regulates serotonin2 receptors in rat brain. Neuropsychopharmacology. 1990; 3:137-148.
11. Ahn H, Makman M. Interaction of LSD and other hallucinogens with dopamine-sensitive adenylate cyclase in primate brain: regional differences. Brain Res. 1979; 162:77-88.
12. Horita A, Hamilton A. Lysergic acid diethylamide: dissociation of its behavioral and hyperthermic effects by DL-alpha-methyl-p-tyrosine. Science. 1969; 164:78-79.
13. Van de Kar L. Neuroendocrine pharmacology of serotonergic (5-HT) neurons. Ann Rev Pharmacol Toxico. 1991; 31: 289-320.
14. Meltzer H, Wiita B, Tricou B, Simonovic M, Fang V. Effects of serotonin precursors and serotonin agonists on plasma hormone levels. In: Ho B, Schoolar J, Usdin E, eds. Serotonin in Biological Psychiatry. New York NY. Raven Press; 1980: 117-139
15. Halaris A, Freedman DX, Fang V. Plasma corticoids and brain tryptophan after acute and tolerance dosage of LSD. Life Sci. 1976; 17:1467-1472
16. Sai-Halasz A, Brunecker G, Szara S. Dimethyltryptamine: ein neues Psychoticum. Psychiatr Neurol. 1958; 135:285-301
17. Horita A, Dille J. The pyretogenic effect of lysergic acid diethylamide. Science. 1954; 120:1110-1111
18. Greiner T, Burch N, Edelberg R. Psychopathology and psychophysiology of minimal LSD-25 dosage: a preliminary dosage-response spectrum. Arch Neurol Psychiatry. 1958; 79:208-210
19. Isbell H. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia. 1959; 1:29-38
20. Fish M, Johnosn N, Horning E. Piptadenia alkaloids: indole bases of P. peregrine (L.) Benth. And related species. J Am Chem Soc. 1955; 77:5892-5895
21. Barker S, Monti J, Christian S. N, N-dimethyltryptamine: an endogenous hallucinogen. Int rev Neurobiol. 1981; 22:83-110
22. Gillin J, Kaplan J, Stillman R, Wyatt R. The psychedelic model of schizophrenia: the case of N, -dimethyltryptamine. Am J Psychiatry. 1976; 133:203-208
23. Strassman R. Human hallucinogenic drug research in the United States: a present day case history and review of the process. J Psychoactive Drug. 1991; 23:29-38
24. Spitzer R, Williams J, Gibbon M. Structured Clinical Interview of DSM-III-R: Outpatient Version. New York, NY: Biometric research Dept, New York State Psychiatric Institute; 1987
25. McKenna D, Peroutka S. Neurochemistry and neurotoxicity of 3, 4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’). J Neurochem. 1990; 54:14-22
26. Price L, Ricaurte G, Krystal J, Heninger G. Neuroendocrine and mood responses to intravenous L-tryptophan in 3, 4-methylenedioxymethamphetamine (MDMA) users. Arch Gen Psychiatry. 1989; 46:20-22
27. Stafford P. Psychedelics Encyclopeida. Rev ed. Boston, Mass: Houghton Miffin Co; 1982:308-331
28. Walker R, Mandel L, Kleinman J, Gillin J, Wyatt R, VandenHeuvel W. Improved selective ion monitoring mass-spectrometric assay for the determination of N, N-dimethyltryptamine in human blood utilizing capillary column gas chromatography. J Chromatogr. 1979;162:539-546
29. Webley G, Mehl H, Willey K. Validation of a sensitive direct assay for melatonin for investigation of circadian rhythms in different species. J Endocrinol. 1985; 106:387-394
30. Angrist B, Gershon S, Sathananthan, Walker R, Lopez-Ramos B, Mandel L, VandenHeuvel W. Dimethyltryptamine levels in blood of schizophrenic patients and control subjects. Psychopharmacology. 1976; 47:29-32
31. Kaplan J, Mandel L, Stillman R, Walker R, VandenHeuvel W, Gillin J, Wyatt R. Blood and urine levels of N, N-dimethyltryptamine following administration of psychoactive doses to human subjects. Psychopharmacologia. 1974; 38:239-245
32. Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N, N-dimethyltryptamine in humans. II: subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994; 51: 98-108
33. Aghajanian G, Foote W, Sheard M. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970; 171: 178-187
34. Deliganis A, Pierce P, Peroutka S. Differential interactions of dimethyltryptamine (DMT) with 5-HT1a and 5-HT2 receptors. Biochem Pharmacol. 1991; 41: 1739-1744
35. Jenner P, Marsden C, Thanki C. Behavioral changes induced by N, N-dimethyltryptamine in rodents. Br J Pharmacol. 1980; 69:69-80
36. Pierce P, Peroutka S. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989; 97:118-122
37. Sasnders-Bush E, Breeding M, Choroid plexus epithelial cells in primary culture: a mode of 5HT1c receptor activation by hallucinogenic drugs. Psychopharmacology 1991; 105:340-346
38. Charig E. Anderson I, Robinson J, Nutt D, Cowen P. L-tryptophan and prolactin release: evidence for interaction between 5-HT1 and 5-HT2 receptors. Hum Psychopharmacol. 1986; 1:93-97
39. Vale W, Spiess J, Rivier C, Rivier J. characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotrophin and _-endorphin. Science. 1981; 213:1394-1396
40. Lewis D, Sherman B. Serotonergic regulation of adrenocorticotropin secretion in man. J Clin Endocrinol Metab. 1984; 58:458-462
41. Petraglia F, Facchinetti F, Martignoni E, Nappi G, Volpe A, Genazzani A. Serotonergic agonists increase plasma levels of _-endorphin and _-lipotropin in humans. J Clin Endocrinol Metab. 1984; 59:1138-1142
42. Anderson I, Cowen P, Grahame-Smith D. The effects of gepirone on neuro-endocrine function and temperature in humans. Psychopharmacology. 1990; 100:498-503
43. Calogero A, Bernardini R, Margioris A, Bagdy G, Gallucci W, Munson P, Tamarkin L, Tomai T, Brady L, Gold P, Chrousos G. Effects of serotonergic agonists and antagonists on corticotrophin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989; 10: 189-200
44. Kato Y, Nakai Y, Imura H, Chichara K, Ohgo S. Effect of 5-hydroxytryptophan (5-HTP) on plasma prolactin levels in man. J Clin Endocrinol Metab. 1874; 38: 695-697
45. Asnis G, Eisenberg J, van Praag H, Lemus C, Friedman J, Miller A. The neuroendocrine response to fenfluramine in depressives and normal controls. Biol Psychiatry. 1988; 24: 117-120
46. Demisch L, Neubauer M. Stimulaiton of human prolactin secretion by mescaline. Psychopharmacology. 1979; 64:361-363
47. Aloi J, Insel T, Mueller E, Murphy D. Neuroendocrine and behavioral effects of mCPP in rhesus monkeys. Life Sci. 1984; 34:1325-1331
48. Smith C, Sare C, Cowen P. Pindolol decreases prolactin and growth hormone responses to intravenous L-tryptophan. Psychopharmacology. 1991; 103:140-142
49. Van de Kar L, Lorens S, Urban J, Bethea C. Effect of selective serotonin (5HT) agonists and 5-HT2 antagonists on prolactin secretion. Neuropharmacology. 1989; 28:299-305
50. Calogero A, Bagdy G, Szemeredi K, Tartaglia M, Gold P, Chrousos G. Mechanisms of serotonin receptor agonist-induced activation of the hypothalamicpituitary-adrenal axis in the rat. Endocrinology. 1990; 126: 1888-1894
51. Snyder SH, Faillace L, Hollister L. 2, 5-Dimethoxy-4 methyl-amphetamine (STP): an new hallucinogenic drug. Science, 1967; 158:669-670
52. Won S, Lin M. 5-Hydroxytryptamine receptors in the hypothalamus mediate thermoregulatory responses in rabbits. Naunyn Schmiedebergs Arch Pharmacol. 1988; 338:256-261
53. Cohen M, Fuller T, Wiley S. Evidence for 5-HT2 receptors mediating contraction in vascular smooth muscle. J Pharmacol Exp Ther. 1981; 21: 421-425
54. Denber H, Merlis S. Studies on mescaline, VI: therapeutic aspects of the mescaline-chlorpromazine combination. J Nerv Ment Dis. 1955; 122: 463-469
55. Turner W, Merlis S. Effect of some indolealkylamines on man. Arch Neurol Psychiatry. 1959; 81:121-129
56. Debire H, Chaouche-Teyara K, Cherqui C, Fournier B, Laubie M, Schmitt H. Characterization of DOI, a putative 5-HT2 receptor agonist in the rat. Eur J Pharmacol. 1989; 168: 369-374
57. Rittenhouse P, Bakkum E, Van de Kar L. Evidence that the serotonin agonist, DOI, increases rennin secretion and blood pressure through both central and peripheral 5-HT2 receptors. J Pharmacol Exp Ther. 1991; 259: 58-65
58. Dreteler G, Wouters W, Saxena P. Systemic and regional hemodynamic effects of the putative 5-HT1A receptor agonist flesinoxan in the cat. J Cardiovasc Pharmacol. 1989; 14:770-776
59. Klee G, Bertino J, Weintraub W, Callaway E III. The influence of varying dosage on the effects of lysergic acid diethylamide (LSD-25) in humans. J Nerv Ment Dis. 1961; 132:404-409
60. Millison D, Haworth S, Rushton A, Wilkinson D, Hobson S, Harry J. The effects of a 5-HT2 antagonist (ICI 169,369) on changes in waking EEG, papillary responses and state of arousal in human volunteers. Br J Clin Pharmacol 1991; 32:447-454
61. Rosenberg D, Isbell H, Miner E, Comparison of placebo, N-dimethyltryptamine and 6-hydroxy-N-dimethltryptamine in man. Psychopharmacology. 1963; 4:39-42
62. Yanai K, Ido T, Ishiwata K, Hatazawa J, Takahashi T, Iwata R, Matsuzawa T. In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N, N-[11C]dimethyltryptamine. Eur J Nuci Med. 1986; 12:141-146
63. Seibyl J, Krystal J, Price L, Woods W, Heninger G, Charney D. 5-HT function in the biochemical and behavioral responses to mCPP in healthy subjects and schizophrenics. Soc Neurosci Abstr. 19889; 15:1236
64. Lowy M, Meltzer H. Stimulation of serum cortisol and prolactin secretion in humans by MK-212, a centrally active serotonin agonist. Biol Psychiatry. 1988; 23:818-828
65. Murphy D, Lesch K, Aulakh C, Pigott T. Serotinon-selective arylpiperazines with neuroendocrine, beharioral, temperature and cardiovascular effects in humans. Pharmacol Rev. 1991; 43:527-552
66. Charney D, Goodman W, Price L, Woods W, Rasmussen S, Heninger G. Serotonin function in obsessive-compulsive disorder. Arch Gen Psychiatry. 1988; 45:177-185
67. Murphy D, Mueller E, Hill J, Tolliver T, Jacobsen F. comparative anxiogenic, neutroendocrine and other physiologic effects of m-chlorophenylpiperazine given intravenously and orally to healthy volunteers. Psychopharmacology. 1989; 98:275-283
68. Lee H, Bastani B, Friedman L, Ramirez L, Meltzer H. Effect of the serotonin agonist, MK-212, on body temperature in schizophrenia. Biol Psychiatry. 1992; 31:460-470
69. Appel JB, Freedman DX. Chemically-induced alterations in the behavioral effects of LSD-25. Biochem Pharmacol. 1964; 13:861-869
70. Nisbet AF, Marsden CA. Increased behavioral response to 5-methoxy-N, N-dimethyltryptamine but not to RU-24969 after intraventricular 5, 7-dihyydroxytryptamine administration. Eur J harmacol. 1984; 104:177-180
71. Ebadi M, Govitrapapong P. Neural pathways and neurotransmitters affecting melatonin synthesis. J Neural Transm. 1986; 21(suppl):125-158
72. Hartley R, Smith J. The activation of pineal hydroxyl-indole-O-methyltransferase by psychotonimetic drugs. J Pharm Pharmacol. 1973; 25:751-752
73. Strassman R, Appenzeller O, Lewy A, Qualls C, Peake G. Increase in plasma melatonin, _-endorphin, and cortisol after a 28.5-mile mountain race; relationship to performance and lack of effect of naltrexone. J Clin Endocrinol Metab. 1989; 148:540-545
74. Price L, Charney D, Delgado P, Heninger G, Serotinin function and depression: neuroendocrine and mood responses to intravenous L-tryptophan in depressed patients and healthy comparison subjects. Am J Psychiatry. 1991; 148:1518-1525
75. Lee M, Meltzer H. Neuroendocrine responses to serotonergic agents in alcoholics. Biol Psychiatry. 1991; 30:1017-1030
76. Fishbein D, Lozovsky D, Jaffe J. Impulsivity, aggression, and neuroendocrine responses to serotonergic stimulation in substance abusers. Biol Psychiatry. 1989; 25:1049-1066
77. Freedman DX. Hallucinogenic drug research: if so, so what? Pharmacol biochem Behav. 1986; 24:407-415